Aeroponics for Spaceflight Plant Growth
J.M. Clawson, A. Hoehn, L.S. Stodieck and P. Todd
BioServe Space Technologies
R.J. Stoner
EnviroGen, Inc., d.b.a. Aeroponics International
Copyright © 2000 Society of Automotive Engineers, Inc.
ABSTRACT
Aeroponics is the process of growing plants in an air/mist environment without the use of soil or an aggregate media. Aeroponics has contributed to advances in several areas of study including root morphology, nutrient uptake, drought and flood stress, and responses to variations in oxygen and/or carbon dioxide root zone concentrations.
The adaptability of the aeroponic process that has benefited researchers make its application to spaceflight plant growth systems appealing. Greater control of growth parameters permit a greater range of crop performance throttling and the elimination of aggregates or common growth substrates lower system mass, lessens disease propagation between plants, and can decrease the required crew time for both planting and harvesting. However, because of the use of less reliable types of aeroponic techniques and concerns with open two-phase fluids within the root zone in microgravity, many advanced life support technology researchers opted for nutrient delivery concepts that offered more fluid control, containment and simpler subsystem designs. The resulting systems have been successful for on-orbit plant growth, but have yet to achieve high-performance crop production equal to terrestrial standards. Re-examining historical concerns, along with an overview of recent work demonstrating hypogravity aeroponics, shows the promise that aeroponics still holds for spaceflight crop production.
INTRODUCTION
The growth of plants during spaceflight poses many challenges to the plant growth system designer, not the least of which is providing nutrients to the roots. As the influence of gravity decreases, surface tension forces dominate fluid behavior. Without a large gravitational driving force, prediction of fluid behavior becomes less intuitive.
Several methods of nutrient delivery have been developed for spaceflight application [Morrow et al., 1992; Dreschel et al., 1994; Heyenga, 1994]. Flow through porous media has been the dominant technology utilized in these systems. While successful in growing plants in orbit, these systems have yet to demonstrate on-orbit
performance equal to terrestrial hydroponic or aeroponic systems.
The focus of spaceflight plant growth appears to be shifting from one of demonstrating capability to one of achieving increased performance. The viability of plant growth systems for the production of crops within a bioregenerative life support system relies heavily on reducing the mass of the cultivation hardware while increasing the yield of the system. By reducing substrates and large solution buffers [Eckart, 1996] while maintaining high plant performance, aeroponic systems continue to demonstrate these abilities, thus remaining a promising technology for spaceflight application.
History
Aeroponics is the process of growing plants in an air/mist environment without the use of soil or an aggregate media. Such conditions occur in nature, as on tropical islands like Hawaii, where orchids develop and grow freely in trees [Rains, 1941]. This form of plant growth remained a natural phenomenon until B. T. Barker succeeded in growing apple trees with a spray [Barker, 1922]. F. W. Went, who in 1957 grew tomatoes and coffee plants with their roots suspended in air using a nutrient mist, termed this air-growing process “aeroponics” [Stoner, 1983].
[sc:videocoursead]
Soon after its development, aeroponics took hold as a valuable research tool. It offered researchers a noninvasive way to examine roots under development. This new technology provided researchers with a larger number and a wider range of experimental parameters to use in their work. Aeroponics has contributed to advances in several areas of study such as root morphology, nutrient uptake, water stress, both drought and flooding, and the root’s response to variations in oxygen and/or carbon dioxide concentrations.
Aeroponics is well suited for the study of root morphology. The absence of aggregates offers researchers easy access to the entire, intact root structure without the damage that can be caused by removal of roots from soils or aggregates. Carter [Carter, 1942] initiated vapor misting of pineapple plants in an enclosed environment to examine the roots following infestation by mealybugs. He found that for studies requiring the examination of the roots, aeroponics reduced the mechanical injury and interference with growth that result from using soil, sand, or even aerated water culture.
The discrete nature of interval/duration aeroponics allows the measurement of nutrient uptake over time under varying conditions. Interval/duration aeroponics involves the intermittent misting of the root zone on periodic intervals for a short duration rather than a constant fogging or misting. Klotz [Klotz, 1944] discovered that vapor/mistgrown plants facilitated his studies of the effects of nutrient concentration and disease in citrus and avocado roots.
Barak et al. [Barak et al., 1996] used an aeroponic system for non-destructive measurement of water and ion uptake rates for cranberries. They found that by measuring the concentrations and volumes of input and efflux solutions, they could accurately calculate the nutrient uptake rate, which was later verified by comparing the results with N-isotope measurements. The ability to precisely control the root zone moisture levels and the amount of water delivered makes aeroponics ideally suited for the study of water stress.
Hubick [Hubick et al., 1982] evaluated aeroponics as a means to produce consistent, minimally water-stressed plants for use in drought or flood physiology experiments. Hubick and Robertson [Hubick et al., 1988; Robertson et al., 1990] went on to study the effects of drought on the transport and metabolism of abscisic acid and root development in aeroponically grown sunflowers by simply altering the interval and duration of the misting for several days.
Some researchers have used aeroponics to study the effects of root zone gas composition on plant performance. Soffer and Burger [Soffer et al., 1988] studied the effects of dissolved oxygen concentrations on the formation of adventitious roots in what they termed “aero-hydroponics.” They utilized the Ein Gedi system, in which three separate zones were formed within the root area. The ends of the roots were submerged in the nutrient reservoir, while the middle of the root section received nutrient mist and the upper portion was above the mist. Their results showed that dissolved O2 is essential to root formation, but went on to show that for the three O2 concentrations tested, the number of roots and root length were always greater in the central misted section than either the submersed section or the un-misted section. Even at the lowest concentration, the misted section rooted successfully.
Alternatively, Yurgalevitch and Janes [Yurgalevitch et al., 1988] used aeroponics to study the effects of root zone CO2 enrichment on tomato seedlings. They found that root zone concentrations of CO2 between 0.5% and 5% significantly stimulated seedling growth.
Conversely, CO2 concentrations of 25% and 50% significantly inhibited growth by as much as 30%.
Du Toit et al. [du Toit et al., 1997] evaluated aeroponics as a screening tool for blight resistance in maize. They praised the aeroponic system as a “valuable, simple, and rapid method for preliminary screening of genotypes for resistance to specific seedling blight or root rot.” The isolating nature of the aeroponic system allowed them to avoid the complications encountered when studying these infections in soil culture. Using ultrasonic misting in a bioreactor, Weathers et al. [Weathers et al., 1988] saw
dramatic increases in development for the micropropagation of plants. Their system has the potential of reducing the costs and increasing the quality of such propagation by producing plants in a third of the time that are healthier and more vigorous.
Aeroponics eventually left the laboratories and entered into the commercial cultivation arena. In 1966, commercial aeroponic pioneer, Bruce Briggs [Briggs, 1966], succeeded in inducing roots on hardwood cuttings by air rooting. He discovered that air-rooted cuttings were tougher and more hardened than those formed in soil and concluded that the basic principle of air-rooting is sound. In 1989, Colorado Power Partners, carried out a comparative study on the number of tomato crop turnarounds (from sprouting or germination to harvesting) that can be achieved with aeroponics [Stoner, 1989].
Researchers determined that when their crops were started from seeds, the first harvestable tomato occurs on
day 68. Fruit could be harvested from these plants until the day 105. With aeroponics, the researchers discovered that vegetative tomato cuttings, consisting of flowers and maturing fruits that are taken from mature tomato plants after 68 days, could be used as a starter crop. These aeroponically grown crops resulted in comparable yields and poundage per plant and allowed 7.7 crop turn-arounds per year compared to 3.5. Eliminating the rock wool bags, conserving water and re-using all effluent nutrients cut additional expenses. Commercial research carried out by Hauser Chemical, Boulder, CO revealed that yew trees could be propagated and grown faster utilizing aeroponics instead of tissue culture [Stoner, 1989]. In this study, the time to vegetatively propagate a 2-inch yew tree was reduced ten fold from 30 to 3 days using aeroponics instead of tissue culture.
Finally, the recent popularization of herbal remedies has spurred the investigation of aeroponics for propagating these supplements. Recent Canadian research has found that the level of the active ingredients in some of these herbs may be manipulated by changing the parameters of the aeroponic nutrient mist [Dufresne, 1999].
Benefits of Aeroponics for Spaceflight CEA
Applications
The adaptability of the aeroponic process that has
benefited both researchers and commercial growers
makes its application to spaceflight plant growth systems
appealing. First, aeroponic technology can apply individual
dosages of various treatments to the plant’s root zone.
Hormones, auxins, growth enhancers, and/or disinfectants
can be individually applied to the roots systems in a onetime,
multiple-time, and/or intermittent application. The
additive-containing effluent can either be recirculated or
diverted. If diverted, the effluent can be discarded or
retained for use in subsequent treatments. Application of
additives with other nutrient delivery technologies can be
more problematic. In hydroponics, for example, a
complete system volume of nutrient solution would require
purging if additives are to be applied into solution and later
rinsed away.
Aeroponics can limit disease transmission since plant-toplant
contact is reduced and each spray pulse can be
sterile. In the case of soil, aggregate, or other media,
disease can spread throughout the growth media,
infecting many plants. In most greenhouses these solid
media require sterilization after each crop and, in many
cases, they are simply discarded and replaced with fresh,
certified sterile media.
A distinct advantage of aeroponic technology is that if a particular plant does become diseased, it can be quickly removed from the plant
support structure without disrupting or infecting the other plants. As an additional safeguard, the aeroponic chamber can easily be cleaned if unsanitary conditions occur by injection of dilute amounts of disinfectants, such as sodium hypochlorite or hydrogen peroxide. The disinfectant effluent can be diverted after application so that continual recirculation of it does not impact plant performance.
Despite all of these cited advantages, plant growth
performance is still of primary importance to both
commercial growers and advanced life support
practitioners alike. Stoner and Clawson [Stoner et al.,
1998] demonstrated that aeroponics compares favorably
in lettuce production per square meter of planting area to
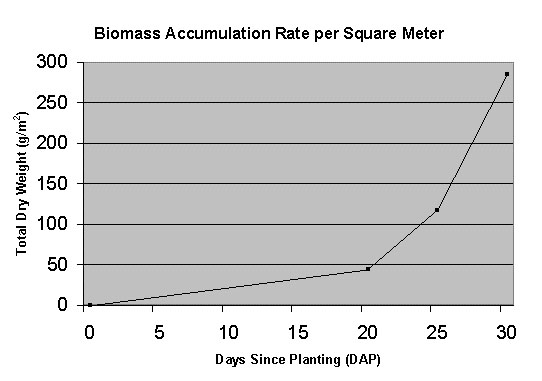
that reported in the advanced life support literature. Figure
1Figure 1 shows the biomass accumulation rate during
their 30-day test.
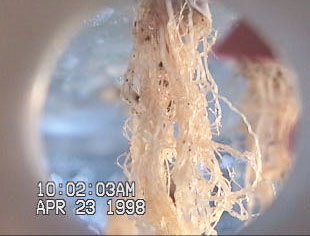
Figure 2Figure 2 demonstrates the affect of substrate on
root biomass development. The thicker substrate (used on
the left plant of Figure 2Figure 2) is thought to retain more
moisture between misting cycles, resulting in reduced
root biomass in early development [Stoner et al., 1998]. Together with modified nutrient solutions, proper selection
of substrate could ultimately increase harvest index.
Figure 1 Lettuce (Waldman’s Green) growth rate per
square meter of growing area achieved by the aeroponic
system during a 30-day test [Stoner et al., 1998].
Open spaces in and around the root systems permit
forced convection, which increases root oxygenation even
at lower pressures such as those found in proposed
inflatable greenhouses. Further, the value of aeroponics as
a research tool for nutrient uptake opens up possibilities
for monitoring of plant health and/or optimization of crops
grown in closed environments. Likewise, variation of root
zone gas concentrations and interval/duration spray
patterns offer yet another opportunity for throttling plant
performance in conjunction with an enclosed plant growth
system.

Figure 2 The effect of substrate on root development. A
thicker, denser germinating substrate was used on the left
treatment while the right treatment was less dense
[Stoner et al., 1998].
Concerns for Spaceflight
The concerns encountered with the use of aeroponics for
spaceflight and/or life support applications can be
separated into two categories, those that are aeroponic
process related and those that are microgravity related.
Aeroponic Process Related Concerns
Tibbitts [Tibbitts et al., 1994] briefly researched
aeroponics and concluded that system failures of his
aeroponic apparatus would result in complete loss of plant
life. In this research, a vaporizer was used to deliver a
continuous mist or fog to the stem and root. With a
continuous fog, the plants become dependant upon the
constant mist and interruption of the fog would soon
cause loss of plant life. The continuous mist can also
contribute to fungal and bacterial growth in the vicinity of
or on the plants. Lastly, due to the fine droplet size and
lower velocity of most continuous fogging systems, some
researchers have encountered difficulty in delivering
nutrients to all the plants when there was a high density of
plants in the chamber [Coston et al., 1983].
Several different approaches have been developed for
providing a nutrient mist in an aeroponic apparatus [Nir,
1982; Schorr et al., 1985; Fraze, 1987; Ehrlich, 1989;
Takayasu, 1989; Rothem, 1992; Keshet et al., 1994;
Ammann, 1998]. However, interval/duration techniques
produce healthier, more natural roots than constant fog or
hydroponic techniques . The interval/duration spray
method conditions the plants to thrive longer on lower
moisture levels and also may reduce pathogen infection.
Roots propagated with interval/duration aeroponics are
much more resistance to interruption of the misting
regimen. Plants have easily survived for a week or more in
a system “safe mode” where lighting intensity and
temperature are lowered and humidity is increased
[Stoner et al., 1998].
In conjunction with the interval/duration method, droplet
size and velocity are also important aeroponic
parameters. These parameters affect the mist collection
efficiency and the depth of penetration of the spray. The
root’s mist collection efficiency depends on its filament
size, drop size, and velocity. For a given filament size and
air velocity, relatively small increase in droplet diameter
will increase the percentage of droplet impingement from
zero to one hundred percent. For example, 1 mm diameter
drops are difficult to impinge. At 30 m/s, a 1 mm drop will
impinge and collect on a 25 mm wire, but not on the larger
diameters of root structures and not at lower velocities.
This is because such a mist can follow the gentler airflow
streamline curvature around larger wires without being
deposited on the wire by momentum carrying it across the
flow lines onto the surface. The higher momentum spray
increases the collection efficiency as well as penetrating
deeper into dense root structures. Another important
benefit of higher spray momentum is that it can dislodge
contaminants to keep the roots clean and healthy.
Microgravity Related Concerns
In validation tests of the Microgravity Plant Nutrient
Experiment (MPNE), performed aboard NASA’s KC-135
microgravity simulator, Dreschel et al. [Dreschel et al.,
1993] briefly studied an aeroponic apparatus. They
reported that in low gravity the fluid in the aeroponic
apparatus tended to form globules that floated around
inside the container and tended to adhere to the corners
of the container. Hoehn [Hoehn, 1998] also studied the
feasibility of both microgravity hydroponics and
aeroponics.
In a series of KC-135 experiments, he
examined the wetting of lettuce roots in microgravity.
Roots were suspended in a nutrient solution and the fluid
dynamics were observed during low and high “g”
maneuvers. Figure 3Figure 3 (top) shows the interaction of
the nutrient solution and the lettuce roots during the
microgravity portion of the aircraft parabola. Figure 3Figure
3 (middle and bottom) shows similar results obtained by
Stoner and Clawson [Stoner et al., 1998] when misting
wheat roots during microgravity parabolas aboard the KC-
135. Additionally, patterns of liquid retention similar to that
seen in microgravity were observed during the parabolas
that simulated Martian and Lunar gravities as well. This
accumulation of solution within and around the root
structure raises concerns with oxygenation of the roots.
Accumulation of solution within other areas of the root
chamber raises concerns with effluent recovery.
In microgravity, the mist droplets impinge on the root
structure and agglomerate first as a water film. If misting
continues, the water film grows into a water “bubble”, as
observed in KC-135 testing [Stoner et al., 1998]. The
degree to which misted droplets collect, or impinge, on
the root structure is again governed by the size and
velocity of the droplet and the root structure upon which it
impinges. Similarly, the size of the “bubble” that forms as
the mist continues to be sprayed depends upon the
velocity of the air/mist flow as it contacts the outer surface
of the accumulated liquid.
[sc:videocoursead]
A 1 mm mist will be too small for wetting roots in the
proposed setting because the necessary impingement
velocities would take too much power and perhaps disturb
root growth. On the other hand, 100 mm diameter droplets
will settle fully out of a gentle airflow in the 1 g of Earth.
The proper mist size for effective impingement occurs with
drop sizes in the 30 mm range. Here, the droplets do not
fall rapidly out of the air stream and will follow the gradual
curvature of pipes and internal surfaces, but will impinge
on root-sized filaments. This means the droplet delivery
process will work about the same way on the ground as in
microgravity.
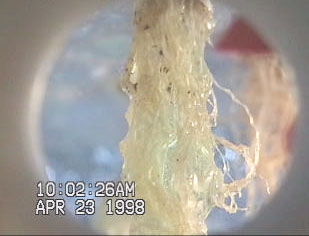
Figure 3 A lettuce root misted in simulated microgravity
(top) and a Wheat root misted at ~1.8 g (middle) and at
microgravity conditions (bottom) [Hoehn, 1998; Stoner et
al., 1998].
Removal of agglomerated liquid in the root structure may
be important to assure adequate oxygenation. During KC-
135 experiments, impingement aeration and aspiration of
the root zone was demonstrated to be an effective method
of removing the liquid from the root structure [Stoner et al.,
1998]. After a spray cycle, the liquid side to the air
atomizing nozzles was interrupted allowing only the air to
impinge on the root. The impinging air stripped off much of
the extraneous liquid clinging to the root.
Once liberated, the liquid can migrate, either staying
airborne or attaching to the walls. Recovery of airborne
effluent can be accomplished with centrifugal separation
methods. A conceptual design of a proposed separator is
shown in Figure.
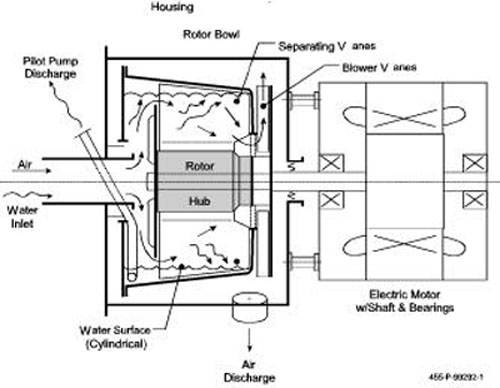
Figure 4. Schematic of a proven two phase
separator/pump [Foster-Miller, 1986].
The centrifuge rotor is supported on the motor shaft, overhung,
enclosed with a housing mounted to the motor. Airwater
mixture, perhaps including liquid slugs, enters the
free end of the rotor, where both phases are deflected
radially by a plate, perhaps having a few “slinger” vanes.
Bulk water is thrown directly to the peripheral pool in the
bowl. Air and droplets flow around the deflector plate into
the mid-section of the bowl. Here a few “swept” vanes
keep the fluids rotating within the bowl while droplets
impinge on them and run outward to the pool. Air flows
inward and then axially through holes in the motor end of
the bowl and is discharged free of water droplets.
The liquid attaching to the walls can be collected via
propellant management devices (PMDs) so called
because of their primary use in the spacecraft propulsion
industry to handle liquid propellants in rigid tanks exposed
to microgravity. In low or zero inertial force environments,
surface tension forces are relatively strong compared to
those of small accelerations. The competition between the
two forces determines the ultimate location of the fluid
that minimizes the sum of the gravitational and surface
energies [Dodge, 1990]. The resultant motion and final
resting configuration of the fluid, although much different
from the motion seen in a gravity field, is predictable, and
PMD’s have been successful in managing fluids in the
microgravity environment.
The use of fine mesh screens is a popular PMD for the
space propulsion industry. Capillary forces drive the liquid
to fill the open spaces in the screen. The resultant surface
tension prevents gas from penetrating the screen barrier.
The use of a screen, however, poses a problem when
employed in the root zone of an aeroponic system. The
root systems of the plants may entangle themselves in
the screen mesh, making harvesting difficult and
complicate equipment preparation for the next crop cycle.
Another method employed for microgravity fluid
management is the vaned PMD. Used on the Viking
spacecraft, a series of vanes protrude radially from a
central standpipe. The vanes provide a surface for the
liquid to wet out onto. The angles formed by the
intersecting vanes allow the fluid to form a curved surface
between them to lower its surface energy state. The
smooth surfaces of the vane type of system are much
more appropriate for the inside of a root chamber.
One offshoot version of the vane PMD involves use of a
contoured surface to form troughs that collect condensate
through drainage holes along the trough. This method was
used by Crowley [Crowley, 1991] in the design of the
condensing surface for an Alkali Metal Thermal to Electric
Conversion Cell (AMTEC). The trough cross section is
formed such that a continuously varying radius of
curvature drives liquid to the bottom of the trough by
surface tension. This is a relatively simple surface
treatment that can be either machined or molded into a
chamber surface. The actual shape of the profile is driven
by the surface tension properties of the fluid.
CONCLUSION
Because of early concerns of open two-phase fluids within
the root zone in microgravity, many advanced life support
technology researchers opted for nutrient delivery
concepts that offered more fluid control, containment and
simpler subsystem designs. The resulting systems have
been successful for on-orbit plant growth, but have yet to
achieve high performance crop production equal to
terrestrial standards.
A re-examination of aeroponics, along with recent work to
demonstrate hypogravity aeroponic technology, shows the
promise that aeroponics still holds for spaceflight crop
production. Technologies for the implementation of a
spaceflight aeroponic system have been shown to exist at
the maturity level needed for direct incorporation into a fullscale
system. Strategies to deal with the effects of the
different gravity environments were explored, and
successful strategies were developed and demonstrated
to maintain the proper parameters for aeroponic plant
growth.
Food production for space mission life support systems requires the optimization of several parameters to make its implementation worthwhile. Aeroponics offers an excellent option for optimizing all of these parameters, such as high total edible biomass production, little or no use of substrates, low power consumption, and minimum effort for planting and harvest, to arrive at a superior plant production system for use in space. The performance of the interval/duration method of aeroponics has been shown to be effective at producing high total edible biomass per area while minimizing the nutrient solution throughput when compared to other systems of similar performance.
ACKNOWLEDGMENTS
This research was supported primarily through the NAS10-98030 contract entitled, “A High Performance,
Gravity Insensitive, Enclosed Aeroponic System for Food Production in Space.”
REFERENCES
1. Ammann, P.R., Jr. (1998). Aeroponic Plant Growth
Apparatus and Method. http://www.patents.ibm.com. United
States: 9.
2. Barak, P., J.D. Smith, A.R. Krueger and L.A. Peterson
(1996). “Measurement of short-term nutrient uptake rates in
cranberry by aeroponics.” Plant, Cell and Environment 19:
237-242.
3. Barker, B.T.P. (1922). Long Ashton Research Station
Annual Report: Studies on root development,
4. Briggs, B.A. (1966). An experiment in air-rooting.
International Plant Propagators’ Society.
5. Carter, W.A. (1942). “A method of growing plants in water
vapor to facilitate examination of roots.” Phytopathology 732:
623-625.
6. Coston, D.C., G.W. Krewer, R.C. Owing and E.G. Denny
(1983). “Air Rooting of Peach Semihardwood Cutting.”
HortScience 18(3): 323.
7. Crowley, C.J. (1991). Condenser design for AMTEC
power conversion. IECEC ’91; Proceedings of the 26th
Intersociety Energy Conversion Engineering Conference, La
Grange Park, IL, AIAA Technical Library.
8. Dodge, F.T. (1990). Fluid Management in Low Gravity.
Low-Gravity Fluid Dynamics and Transport Phenomena. J. N.
Koster and R. L. Sani. Washington, D.C., American Institute of
Aeronautics and Astronautics, Inc. 130: 3-13.
9. Dreschel, T.W., C.S. Brown, W.C. Piastuch, C.R. Hinkle
and W.M. Knott (1994). “Porous Tube plant nutrient delivery
system development: A device for nutrient delivery in
microgravity.” Advances in Space Research 14(11): (11)47-
(11)51.
10. Dreschel, T.W., C.W. Carlson, H.W. Wells, K.F. Anderson,
W.M. Knott and W. Munsey (1993). Physical Testing for the
Microgravity Plant Nutrient Experiment. 1993 International
Summer Meeting, Spokane, WA, American Society of
Agricultural Engineers.
11. du Toit, L.J., H.W. Kirby and W.L. Pedersen (1997).
“Evaluation of an Aeroponics System to Screen Maize
Genotypes for Resistance to Fusarium graminearum
Seedling Blight.” Plant Disease 81(2): 175-179.
12. Dufresne, S. (1999). Herbal GreenHouses, Inc.
13. Eckart, P. (1996). Spaceflight Life Supprt and
Biospherics. Torrance-Dordrecht/Boston/London, Microcosm
Press-Kluwer Academic Publishers.
14. Ehrlich, K.F. (1989). Aeroponic Apparatus.
http://www.patents.ibm.com. United States: 10.
15. Foster-Miller, I. (1986). Two-Phase Separating Pump
16. Fraze, R.E. (1987). Plant Propagation System and
Apparatus. http://www.patents.ibm.com. United States: 12.
17. Heyenga, A.G. (1994). “Application of a water replenished
solidified nutrient media support system in long term
cultivation of wheat.” AGSB Bulletin 8(1): 40 (abstr).
18. Hoehn, A. (1998). Root Wetting Experiments aboard
NASA’s KC-135 Microgravity Simulator
19. Hubick, K.T., D.R. Drakeford and D.M. Reid (1982). “A
comparison of two techniques for growing minimally waterstressed
plants.” Canadian Journal of Botany 60: 219-223.
20. Hubick, K.T. and D.M. Reid (1988). “Effect of drought on
transport and metabolism of abscisic acid in aeroponically
grown Helianthus annus.” Physiologio Plantarum 74: 317-
325.
21. Keshet, A. and Y. Shoham (1994). Fog Generator.
http://www.patents.ibm.com. United States, Shira Aeroponics,
Ltd.: 10.
22. Klotz, L.G. (1944). “A simplified method of growing plants
with roots in nutrient vapors.” Phytopathology 34: 507-508.
23. Morrow, R.C., R.J. Bula, T.W. Tibbitts and W.R. Dinauer
(1992). A matrix-based porous tube water and nutrient
delivery system. Proceedings of the 22nd International
Conference on Environmental Systems, Seattle, WA., SAE.
24. Nir, I. (1982). Apparatus and Method for Plant growth in
Aeroponic Conditions. http://www.patents.ibm.com. United
States, ADI-Aeroponics Growth, Ltd.: 21.
25. Rains, M.A. (1941). “Methods of growing plants in water
and air.” Torreya 41: 103-104.
26. Robertson, J.M., K.T. Hubick, E.C. Yeung and D.M. Reid
(1990). “Developmental responses to drought and abscisic
acid in sunflower roots. 1. Root growth, apical anatomy, and
osmotic adjustment.” Journal of Experimental Botany 41: 325-
337.
27. Rothem, T. (1992). System for germination, propagation
and growing plants in utrasonic-fog conditions (aeroponics).
US.
28. Schorr, S. and R. Stoner (1985). Methods and Apparatus
for Aeroponic Growing of Plants. United States.
29. Soffer, H. and D.W. Burger (1988). “Effects of dissolved
oxygen concentrations in aero-hydroponics on the formation
and growth of adventitious roots.” Journal of the American
Society for Horticultural Science 113(2): 218-221.
30. Stoner, R. (1989). Unpublished data on the number of
crop turnarounds available with aeroponic tomato
propagation.
31. Stoner, R. and J.M. Clawson (1998). A High Performance,
Gravity Insensitive, Enclosed Aeroponic System for Food
Production in Space, Phase I SBIR Final Report, NAS10-
98030 EnviroGen, Inc.
32. Stoner, R.J. (1983). “Aeroponics Versus Bed and
Hydroponic Propagation.” Florists’ Review 173(4477).
33. Stoner, R.J. (1989). Aeroponic Taxus Growth Experiment,
Internal Report, Hauser Chemical
34. Takayasu, M. (1989). Aeroponic Apparatus.
http://www.patents.ibm.com. United States: 9.
35. Tibbitts, T.W., W. Cao and R.M. Wheeler (1994). Growth
of Potatoes for CELSS, NASA Contractor Report 177646
36. Weathers, P.J., R.D. Cheetham and K.L. Giles (1988).
“Dramatic Increases in Shoot Number and Lengths for Musa,
Cordyline, and Nephrylepsis using Nutrient Mists.” Act
Hortaculturae 230: 39-44.
37. Yurgalevitch, C.M. and H.W. Janes (1988). “Carbon
dioxide enrichment of the root zone of tomato seedlings.”
Journal of Horticultural Science 63: 265-270.
DEFINITIONS, ACRONYMS, ABBREVIATIONS
AMTEC Alkali Metal Thermal to Electric Conversion Cell
CO2 Carbon Dioxide
MPNE Microgravity Plant Nutrient Experiment
O2 Oxygen
PMD Propellant Management Device